Dr. Xueqian Zhuang (PhD) and the research group of Dr. Tuomas Tammela (MD, PhD) at Memorial Sloan Kettering Cancer Center (MSKCC) are redefining our understanding of how aging impacts cancer. By utilizing Aiforia® Create to automate complex histopathological grading, the team uncovered a surprising tumor-suppressive role of the aged cell of origin in lung cancer.
At MSKCC, Dr. Tammela’s lab investigates the phenotypic heterogeneity of cancer cells. Their goal is to identify pathways and physiological conditions that drive distinct cellular phenotypes to develop new therapies that reduce tumor cellular heterogeneity. To achieve this, the lab uses a multifaceted approach that includes genetically engineered mouse models, single-cell approaches, lineage-tracing, CRISPR-mediated gene regulation, and advanced imaging techniques. Alongside a vast array of research methods, the team has integrated AI-powered histological image analysis into their high-tech scientific toolbox to pace the research.
Flipping the script on how age matters for cancer
In May 2025, Dr. Xueqian Zhuang et al. published a study, Ageing limits stemness and tumorigenesis by reprogramming iron homeostasis, in Nature magazine, revealing that aging imposes a tumor-suppressive restraint by intrinsically limiting the stemness of AT2 (Alveolar Type II) cells, the cell of origin in lung cancer. This finding challenges the traditional view that aging only promotes cancer.
While much is known about how an aged microenvironment can promote metastasis, our understanding of how aging impacts the cell of origin, the healthy cell where cancer first starts, has been limited. “To tackle this question, we used genetically engineered mouse models of lung adenocarcinoma, where we can incorporate the physiology of aging in both the cell of origin and microenvironment and recapitulate the full spectrum of tumor development, in particular the early tumorigenesis process,” Dr. Zhuang explains. This allowed the team to recapitulate the etiology of human lung cancer in the context of aging.
However, analyzing these cancer models requires meticulous grading of hundreds of primary tumors. Manual grading is not only time-consuming but also prone to the "general impression bias" of the human eye, creating a need for a more objective and scalable solution.
How does AI improve the accuracy in tumor analysis?
AI improves lung cancer grading by removing the subjectivity inherent in manual visual assessment. AI models trained on thousands of data points provide standardized, reproducible analysis of histological features. This ensures that every tumor is objectively evaluated against the same criteria, leading to greater precision than with traditional manual methods.
To achieve this high standard of accuracy in tumor analysis, Dr. Zhuang used Aiforia® Create to develop an AI model specifically for the histopathological grading of mouse lung adenocarcinoma (LUAD).
By adopting a "human-in-the-loop" workflow, the researchers maintained oversight by manually counting individual tumors, while AI handled the intensive, objective grading. The coordination between human expertise and machine precision allowed the Tammela Lab to scale the research without compromising on details.
“The efficiency and precision of Aiforia® analysis is superb and consistent. A murine cancer pathologist is a privilege only available for some institutes, but Aiforia® makes this level of expertise accessible regardless of location,” says Dr. Zhuang.
The unexpected role of the aged cell of origin
The study’s most striking finding was that while the aged microenvironment is technically more permissive for tumor initiation, the tumors themselves grew less. By investigating the cell of origin, the team discovered that aging triggers the induction of the transcription factor NUPR1. This induction leads to functional iron insufficiency, which in turn causes a loss of stemness in aged alveolar cells, the cell of origin in lung cancer. Essentially, aging creates a cell-intrinsic barrier that suppresses the tumors that the surrounding tumor microenvironment (TME) is trying to promote.
“The efficiency and precision of Aiforia® analysis is superb and consistent.” – Xueqian Zhuang, PhD, Memorial Sloan Kettering Cancer Center
Crucially, the age-specific gene signatures identified in these mouse models were predictive of patient age in human lung cancer cases. “These findings demonstrate that aging fundamentally changes the biology of lung cancer, shedding light on novel therapeutic modulation of cellular iron homeostasis or ferroptosis in regenerative medicine and in cancer prevention for the elderly population,” Dr. Zhuang unravels. This highlights how AI-elevated research can uncover conserved biological mechanisms.
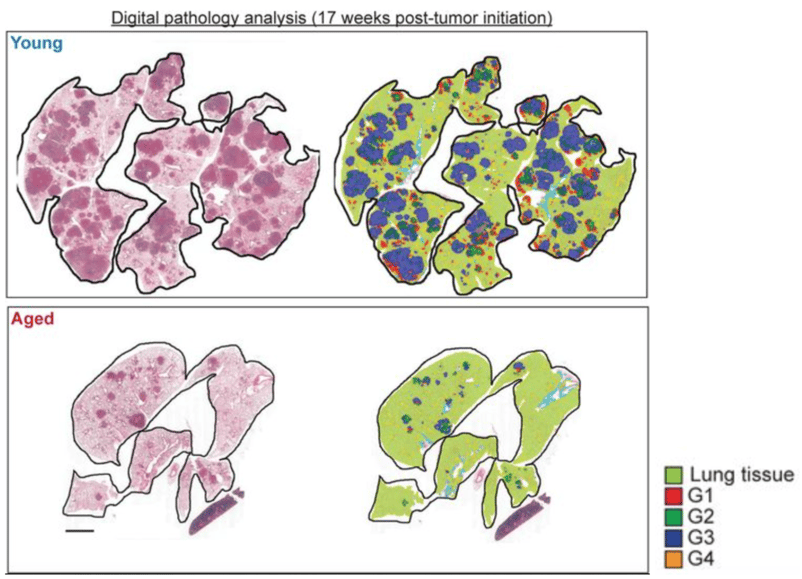
Histopathological grading of KP LUAD tumors in aged vs. young mice at 12 and 17 weeks post-tumor initiation.
Scaling research capabilities through accessible AI tools
For the Tammela Lab, the integration of AI was about more than just speed. It was about method standardization. In a research setting where different researchers may use slightly different subjective grading scales, the Aiforia® Platform provides a digital gold standard.
The team also highlighted the value of Aiforia’s support. During the project, when an inconsistent H&E stain threatened the analysis, the Aiforia support team quickly identified the issue, allowing the researchers to re-stain and continue their work without having to build an entirely new algorithm from scratch.
Future directions
The team at MSKCC plans to continue investigating the role of iron homeostasis in lung tumorigenesis. Their next research target? Using AI to decode the TME, especially the organization of the immune cells in the TME. "This will reveal functional insights into the complex interactions between immune cells and cancer cells that were previously too complex to quantify manually," Dr. Zhuang predicts.