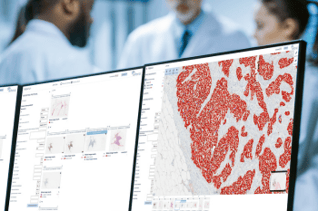
Aiforia® Breast Cancer Suite
Aiforia® Breast Cancer Suite consists of AI models with an optimized interactive user interface that supports pathologists in the histological grading of breast cancer and in the assessment of breast cancer IHC markers.
- Aiforia® Breast Cancer Grading
- Aiforia® Breast Cancer ER
- Aiforia® Breast Cancer PR
- Aiforia® Breast Cancer HER2
- Aiforia® Breast Cancer Ki67
- Aiforia® Lymph Node Metastasis
All AI models in Aiforia® Breast Cancer Suite are CE-IVD marked for diagnostic use in EU and EEA countries and for Research Use Only (RUO) and Performance Studies Only (PSO) in all other market areas.
.svg)
.svg)
.svg)
.svg)
.svg)
.svg)